Algae which are associated with third generation have advantageous characteristics that can be more efficient and sustainable compared to first and second generation biofuels.
Pretreatment of third generation has gained attention. The addition of enzymes and acids along with alterations of the physical environment (i.e. temperature or pH) can optimise the pretreatment stage.
This paper will discuss studies done on macroalgae Ulva Lactua as well as the microalgae Asterarcys quadricellulare.
Fourth generation uses genetic modifications to optimise biomass extraction. Technologies such as the Clustered Regularly Interspaced Short Palindromic Repeats System were used in mentioned studies to alter genomes of the yeast Saccharomyces cerevisiae.
Mutagenesis was also part of a study which was utilised to weaken the cell wall of the microalgae Chlorella vulgaris.
Commercialisation has not been seen in these two generations, but their advances in research can be promising for future production. This paper will discuss recent advances specifically on third-and fourth-generation bioethanol.
Introduction
Bioethanol has been around for centuries. With the first industrial applications in the 19th century, the renewable source originated using food crops such as sugar cane [1].
Bioethanol depends on fermentation, utilising sugar from biomass [2]. Referring to Figure 1, bioethanol can be categorised into four generations [3].
First generation extracts sugar through food crops, second generation uses non-edible crops, third generation focuses on algae, and the fourth and newest generation is not using different organisms but genetically modifying them [1].
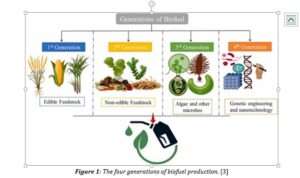
The first generation is the main source of commercialised production.
As efficient as the first generation is, the crops can be competition to food security [1].
Second generation is also at the commercialisation level, but it faces challenges due to their complex structure [2].
Second generation have lignocellulosic structures that are composed of cellulose, hemicellulose, and lignin [4].
The composition and structure of the cell wall causes challenges with hydrolysis, a key factor in the pretreatment step of production [4].
Without this crucial step, sugar is difficult to extract. Another concern towards first and second generation is land use.
Although the crops harvested for second generation do not have value towards food consumption, it does require land that could be used towards edible feedstock.
Third and fourth generation can offer solutions to limitations the other two generations face.
Third generation
Algae have gained traction in the past few years. Although not commercialised, third generation has characteristics that are beneficial for high-scale production. This includes minimal resources and flexibility in a variety of environments [5]. Algae have an advantageous quality that first and second generation lack.
That is, their carbon sequestration capabilities. Along with their beneficial composition of carbohydrates and lipids, they also contribute to sequestration of CO2[6]. Algae can be categorised into two groups, macroalgae and microalgae [1].
Macroalgae cultivation
Macroalgae are also known as seaweed. It can be classified as Chlorophyceae (green seaweed), Phaeophyceae (brown seaweed) and Rhodophyceae (red seaweed) [2].
Macroalgae is found in marine environments, but it can be cultivated in a range from controlled saline water to seawater allowing offshore and onshore farming to be feasible.
For Phaeophyceae alone, 3% of global coastlines have an estimated 200 billion liters of biofuel that can be produced [7].
The seaweed can be grown as kelp systems, utilising ropes and weights to compose a vertical, horizontal, or grid arrangement. Algae grown using this technique can grow 20-30 times faster than livestock crops [5].
The majority of macroalgae is grown onshore such as Figure 2 [8].

This method is responsible for 90% of production, due to fewer structures needed to keep lines in place as well as lower marine life interference [7].
Seaweed can also be integrated to benefit aquacultures. Combined Aquaculture Multi-Use Systems can be seen in many fish or shrimp farms in Canada. Native seaweeds like Macrocystis Pyrifera can remove up to 75% of nutrient effluents [7]. These integrated systems can ultimately result in more sustainable cultivation.
For the harvesting of macroalgae, their plant-like structures allow for similar harvesting methods to be done.
Manual techniques such as nets or sickles can be used in this process. Mechanical methods employ harvesters like dredge cutters and can be seen attached to boats. Manual harvesting is most used but the viability for mechanical is promising for commercialisation [2].
Microalgae cultivation
Microalgae are single-celled organisms. Their high storage capabilities of proteins and starch improve ethanol production [9].
These organisms also offer a benefit to the environment as well as reducing greenhouse gas emissions. Both eukaryotic and prokaryotic are critical to aquatic and freshwater ecosystems. This is due to their photosynthetic efficiency [10]. Microalgae can also clean water. In bodies with high pollutants and nutrients such as wastewater, microalgae can thrive.
Conventional wastewater treatment can be a combination of chemical reactions with biological aspects; microalgae can be utilised as the biological component. The algae can consume contaminants and be used for growth. [10].
This can result in benefits on both parts, the water will be cleansed of contaminants and excess nutrients, while the microalgae communities can flourish and have high cultivation.
Applications of microalgae using this route would be considered open-pond systems.
While it offers uses of wastewater, its productivity can be inconsistent due to fluctuations such as temperature and contamination levels [9].
Photobioreactors can be used for closed systems and can promote higher production of microalgae. Simulations such as one done by Wang et al., studied alterations on the photobioreactor, specifically the optimal incline of baffles on a photobioreactor [11].
The optimal performance had an algal biomass increase of 24.11%–33.02% and a carbon fixation rate of 106% increase. While the machinery has promising results, it can be quite costly and may not have a valuable rate of return [11].
Processing advances
The processing of first and second generation differs significantly from third generation [12].
Limitations that third generation bioethanol faces stem from the lack of research and technology geared towards algae [9].
Recently, there has been a focus towards their biomass processing. The pretreatment process is crucial in extracting biomass. This step can improve the accessibility of carbohydrates [13].
An array of methods can be used in pretreatment and because of this, hybrid pretreatments are often used which are the combination of methods such as incorporating both chemical and biological treatments.
Genhiah et al., used the macroalgae Ulva Lactuca to research what alterations in bioethanol production can be altered for optimisation.
It was found that sulfuric acid can create an optimised reducing sugar concentration.
The optimal percentage was found at 4% with roughly 10.52 mg/mL [12]. Carbohydrate yield can also be increased with temperature.
A variation in temperature 80-120 °C during the pretreatment process found that carbohydrate yield was optimal at 100 °C.
A similar conclusion can be drawn for microalgae. The strain Asterarcys quadricellulare was used by S. Karthikeyan to study optimisation.
When acids H2SO4 and HCL were used for hydrolysis, it was found that the optimised glucose production was using H2SO4.
This variation was processed for 180 minutes with a H2SO4 concentration of 2% and temperature of 100 °C.
The result was 308.38 milligrams of glucose per gramme of biomass. This was 41.68 mg higher than the lowest yield which had the same conditions but a 0.5% H2SO4 concentration [6].
The introduction of enzymes to the pretreatment can also have a considerable influence on reducing sugar concentration.
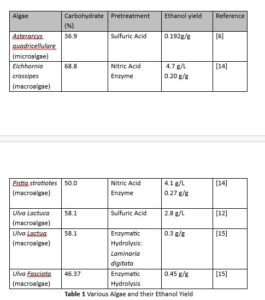
A study done by Jelani et al., found that incorporating commercial enzymes into the pretreatment process can improve the reducing sugar concentration significantly. The four macroalgae used all saw increases in concentration [14].
As Table 1 shows, pretreatment methods all have different results. Even when the same treatments are used, yields can vary significantly between species.
Fourth generation
The newest addition to bioethanol production involves genetic alterations. This is also known as fourth generation [16].
This approach can have an influence on all the other generations by utilising alterations in bacteria, algae and even yeast and modifying these organisms to optimise bioethanol production along with other characteristics such as carbon sequestration.
Yeast plays a vital role in bioethanol production. As the host organism, researching how to optimise their role can drastically improve production.
The most used yeast Saccharomyces cerevisiae has been prevalent due to its ability to withstand a range of conditions and reliability within the industry [17]. Although this yeast has useful characteristics, limitations such as low stress tolerance are limiting their capabilities [17].
The use of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) systems, which changes an organism’s DNA by altering their genomes has recently been used as a toll for optimisation [18].
Use of CRISPR-Cas9 on Saccharomyces cerevisiae shows promising potential to alter the yeast’s efficiency. Due to its tendency for homologous recombination for DNA repair, making changes like removing or altering genes to the yeast’s DNA can be easily applicable [17].
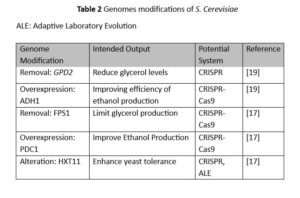
Table 2 illustrates genome modifications along with their purpose. A study done by Yang et al. used CRISPR-Cas9 to remove genes GPD2, FPS1 and ADH2.
The results showed an overall increase in efficiency of 0.18% [19].
Byproduct levels were also measured after the fermentation process. One of which showed a significant decrease in glycerol when the genomes GPD2 and FPS1 were removed.
Lower levels of glycerol are ideal for ethanol production as it indicates more carbon was used toward ethanol production rather than byproducts [20].
Another pathway fourth generation has taken is mutagenesis.
Although this method isn’t as precise as genome modifications, recent studies have shown its potential [21].
Canteri et al., modified the cell wall of microalgae Chlorella vulgaris (chlorella v.) [22].
Its robust structure has limited the algae’s capabilities in terms of biomass extraction. The algae went through two rounds of mutagenesis.
In the first round, the electron density layer was lower in four out of the six mutated strains. However, the biomass extraction was left unchanged.
The second round only had two strains but it gave exciting results.
The resistance of the cell wall weakened. Ultimately, the biomass extraction doubled in comparison to the wild strain [21].
Despite its lack of precision, mutagenesis has optimistic capabilities when the right mutation occurs.
Conclusion
As energy consumption increases and fossil fuels deplete, it is critical to focus on ways to improve renewable energy [6].
Recent studies of bioethanol can have a useful impact in the future. The bioethanol industry is still growing and becoming more prominent but only first and second generation are commercialised [23].
Although third and fourth generation are still in the research stages, they show potential.
Countries such as Brazil or the US that incorporate bioethanol as an energy source, may find algae to be just as beneficial as other biomasses [23].
For third generation, algae have beneficial components that can help other industries, water systems and the environment.
Incorporation of acids and enzymes for algae-based ethanol has shown improvements in extraction and carbon sequestration [6,14,15].
Fourth generation, utilising addition of genetically modified organisms into bioethanol, is also optimistic. Alteration in genomes of yeast allows specific adjustments that can facilitate the fermentation process on a case-to-case basis [17,19].
The mutagenesis of algae has also been shown to be promising. Through random mutation, different algae strains have led to optimal productivity [22].
With more research, third and fourth generation can become more prominent in bioethanol production and the energy sector.
About the authors
Dr. Raj Shah, is a Director at Koehler Instrument Company in New York, where he has worked for the last 25 plus years. He is an elected Fellow by his peers at ASTM, IChemE, ASTM, AOCS, CMI, STLE, AIC, NLGI, INSTMC, Institute of Physics, The Energy Institute and The Royal Society of Chemistry. An ASTM Eagle award recipient, Dr. Shah recently coedited the bestseller, Fuels and Lubricants handbook, details of which are available at ASTM’s Long-awaited Fuels and Lubricants Handbook https://bit.ly/3u2e6GY. He earned his doctorate in Chemical Engineering from The Pennsylvania State University and is a Fellow from The Chartered Management Institute, London.
Dr. Shah is also a Chartered Scientist with the Science Council, a Chartered Petroleum Engineer with the Energy Institute and a Chartered Engineer with the Engineering council, UK. Dr. Shah was recently granted the honorific of “Eminent engineer” with Tau beta Pi, the largest engineering society in the USA.
He is on the Advisory board of directors at Farmingdale university (Mechanical Technology), Auburn Univ (Tribology), SUNY, Farmingdale, (Engineering Management) and State university of NY, Stony Brook (Chemical engineering/ Material Science and engineering). An Adjunct Professor at the State University of New York, Stony Brook, in the Department of Material Science and Chemical Engineering, Raj also has over 725 publications and has been active in the energy industry for over 3 decades. More information on Raj can be found at https://shorturl.at/mgs42
Ms. Madeline Chiappone is a part of a thriving internship program at Koehler Instrument company in Holtsville and is a Chemical and Molecular Engineering Undergraduate Student from Stony Brook University, Stony Brook, New York, where Dr. Shah is the chair of the external advisory board of directors.
References
[1] Khairati, M. (2024). Issue: 8. International Journal of Research and Review (Ijrrjournal.com), 11(8). https://doi.org/10.52403/ijrr.20240860
[2] Müller, C., Scapini, T., Rempel, A., Abaide, E. R., Camargo, A. F., Nazari, M. T., Tadioto, V., Bonatto, C., Tres, M. V., Zabot, G. L., Colla, L. M., Treichel, H., & Alves, S. L. (2023). Challenges and opportunities for third-generation ethanol production: A critical review. Engineering Microbiology, 3(1), 100056. doi.org/10.1016/j.engmic.2022.100056
[3] Kazmi, A., Sultana, T., Ali, A., Nijabat, A., Li, G., & Hou, H. (2025). Innovations in bioethanol production: A comprehensive review of feedstock generations and technology advances. Energy Strategy Reviews, 57(101634), 101634. doi.org/10.1016/j.esr.2024.101634
[4] Zoghlami, A., & Paës, G. (2019). Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Frontiers in Chemistry, 7. doi.org/10.3389/fchem.2019.00874
[5] V, G. S., M, D. K., Pugazhendi, A., Bajhaiya, A. K., Gugulothu, P., & J, R. B. (2021). Biofuel production from Macroalgae: present scenario and future scope. Bioengineered, 12(2), 9216–9238. doi.org/10.1080/21655979.2021.1996019
[6] Karthikeyan, S. (2023). Large Scale Cultivation and Pretreatment Optimization of Freshwater Microalgae Biomass for Bioethanol Production by Yeast Fermentation. Nature Environment and Pollution Technology, 22(2), 1035–1040. doi.org/10.46488/nept.2023.v22i02.051
[7] Kouhgardi, E., Zendehboudi, S., Mohammadzadeh, O., Lohi, A., & Chatzis, I. (2023). Current status and future prospects of biofuel production from brown algae in North America: Progress and challenges. Renewable and Sustainable Energy Reviews, 172, 113012. doi.org/10.1016/j.rser.2022.113012
[8] Wiedenhoft, H. (2019, November 20). Seaweed farming gains traction. Aquaculture North America. aquaculturenorthamerica.com/seaweed-farming-gains-traction/
[9] Muhammad Abdullah, Zain Ali, Muhammad Talha Yasin, Kinza Amanat, Fatima Sarwar, Jallat Khan, Khurshid Ahmad. (2024). Advancements in sustainable production of biofuel by microalgae: Recent insights and future directions, Environmental Research, Volume 262, Part 2, 2024, doi.org/10.1016/j.envres.2024.119902.
[10] Peter, Angela & Khoo, Kuan Shiong & Kit Wayne, Chew & Ling, Tau & Ho, Shih-Hsin & Chang, Jo-Shu & Show, Pau-Loke. (2021). Microalgae for biofuels, wastewater treatment and environmental monitoring. Environmental Chemistry Letters. 19. 10.1007/s10311-021-01219-6.
[11] Wang, L., Zhao, R., Wang, Q., Han, Z., & Mao, X. (2022). Novel bioreactor with inclined baffles in cost-efficiently increasing algal biomass and carbon fixation. Energy, 247, 123453. doi.org/10.1016/j.energy.2022.123453
[12] Kalavathy Gengiah, Naveenkumar Rajendran, Khalid A. Al-Ghanim, Marimuthu Govindarajan, Baskar Gurunathan. (2024). Process and technoeconomic analysis of bioethanol production from residual biomass of marine macroalgae Ulva lactuca, Science of The Total Environment, doi.org/10.1016/j.scitotenv.2023.161661
[13] Jain, S., & Kumar, S. (2024). A comprehensive review of bioethanol production from diverse feedstocks: Current advancements and economic perspectives. Energy (Oxford), 296, 131130–131130. doi.org/10.1016/j.energy.2024.131130
[14] Jelani, F., Walker, G. & Akunna, J. Effects of thermo-chemical and enzymatic pre-treatment of tropical seaweeds and freshwater macrophytes on biogas and bioethanol production. Int. J. Environ. Sci. Technol. 20, 12999–13008 (2023). doi.org/10.1007/s13762-023-04843-7
[15] Qarri, A., & Israel, A. (2020). Seasonal biomass production, fermentable saccharification and potential ethanol yields in the marine macroalga Ulva sp. (Chlorophyta). Renewable Energy, 145, 2101–2107. doi.org/10.1016/j.renene.2019.07.155
[16] Cavelius, P., Engelhart-Straub, S., Mehlmer, N., Lercher, J., Awad, D., & Brück, T. (2023). The potential of biofuels from first to fourth generation. PLoS biology, 21(3), e3002063. doi.org/10.1371/journal.pbio.3002063
[17] Yook, S., & Alper, H. S. (2025). Recent advances in genetic engineering and chemical production in yeast species. FEMS yeast research, 25, foaf009. doi.org/10.1093/femsyr/foaf009
[18] National Library of medicine. (2022, March 22). What Are Genome Editing and CRISPR-Cas9? Medlineplus; National Library of Medicine. medlineplus.gov/genetics/understanding/genomicresearch/genomeediting/
[19] Yang, P., Jiang, S., Jiang, S., Lu, S., Zheng, Z., Chen, J., Wu, W., & Jiang, S. (2022). CRISPR-Cas9 Approach Constructed Engineered Saccharomyces cerevisiae with the Deletion of GPD2, FPS1, and ADH2 to Enhance the Production of Ethanol. Journal of fungi (Basel, Switzerland), 8(7), 703. doi.org/10.3390/jof8070703
[20] Mutton, M. J. R., Ferrari, F. C. S., Freita, L. A., Freita, C. M., Andrietta, M. D. G. S., & Andrietta, S. R. (2019). Interaction between the production of ethanol and glycerol in fed-batch bioreactors. Brazilian journal of microbiology : [publication of the Brazilian Society for Microbiology], 50(2), 389–394. doi.org/10.1007/s42770-019-00051-z
[21] Xin, Z. (2023). Mutagenesis in the Age of Next-Generation-Sequencing and Genome Editing. Plants, 12(19), 3403. doi.org/10.3390/plants12193403
[22] Paolo Canteri, Battarra, C., Giulia Mandalà, Monti, F., Bellini, E., Hidasi, N., Zeno Guardini, Ferrari, S., Bassi, R., & Luca Dall'Osto. (2025). Chlorella vulgaris mutants with altered cell walls show increased permeability and enhanced extractability of intracellular molecules. Biotechnology for Biofuels and Bioproducts, 18(1). doi.org/10.1186/s13068-025-02663-0
[23] Ramsey, S. (2023). Fuel Ethanol Use Expanding Globally but Still Concentrated in Few Markets | Economic Research Service. Usda.gov. ers.usda.gov/amber-waves/2023/june/fuel-ethanol-use-expanding-globally-but-still-concentrated-in-few-markets














